Laboratory
Astrophysics Research Group
|
|
|
The
Fast-Ion-Beam Laser Lab at Western |
![]() Astronomy needs atomic data:
Astronomy needs atomic data:
![]() Astrophysicists
study the process of nucleosynthesis
in which the elements are created by nuclear reactions in stars and
supernovas. The elements beyond 56Fe,
the most stable nucleus, are produced by neutron capture. In regions of intense neutron flux
(supernovas) the “rapid” r-process creates unstable neutron-rich nuclei which
beta decay to more stable isotopes, while the rare p-process creates
proton-rich isotopes by photodisintegration.
In less intense regions the “slow” s-process produces a sequence of
relatively stable isotopes. The
lanthanide elements (rare earths) are particularly suited to unraveling the
history of nucleosynthesis. Samarium, for example, has seven stable
isotopes: 148Sm and 150Sm
are produced by the s-process, 144Sm by the p-process, 154Sm
by the r-process, and 147Sm, 149Sm, and 152Sm
by both the r- and s-processes. These
studies require that the abundances of the elements be determined from
observations.
Astrophysicists
study the process of nucleosynthesis
in which the elements are created by nuclear reactions in stars and
supernovas. The elements beyond 56Fe,
the most stable nucleus, are produced by neutron capture. In regions of intense neutron flux
(supernovas) the “rapid” r-process creates unstable neutron-rich nuclei which
beta decay to more stable isotopes, while the rare p-process creates
proton-rich isotopes by photodisintegration.
In less intense regions the “slow” s-process produces a sequence of
relatively stable isotopes. The
lanthanide elements (rare earths) are particularly suited to unraveling the
history of nucleosynthesis. Samarium, for example, has seven stable
isotopes: 148Sm and 150Sm
are produced by the s-process, 144Sm by the p-process, 154Sm
by the r-process, and 147Sm, 149Sm, and 152Sm
by both the r- and s-processes. These
studies require that the abundances of the elements be determined from
observations.
![]() Stellar
astrophysicists can only study stellar interiors indirectly by observing the
visible outer surface, called the photosphere.
In stars like our Sun, the abundance distribution of the chemical
elements is representative of the bulk abundance in the whole star; however, in
the “chemically peculiar” CP stars, the surface abundances can differ by orders
of magnitude from the bulk abundances.
This chemical fractionation is produced by radiation pressure selectively
driving certain species of atoms toward the surface. In some stars, convection and turbulent
mixing restores a homogenous distribution, but not in CP stars; thus the
surface abundances contain valuable information on what is happening in the unseen
interior of the star.
Stellar
astrophysicists can only study stellar interiors indirectly by observing the
visible outer surface, called the photosphere.
In stars like our Sun, the abundance distribution of the chemical
elements is representative of the bulk abundance in the whole star; however, in
the “chemically peculiar” CP stars, the surface abundances can differ by orders
of magnitude from the bulk abundances.
This chemical fractionation is produced by radiation pressure selectively
driving certain species of atoms toward the surface. In some stars, convection and turbulent
mixing restores a homogenous distribution, but not in CP stars; thus the
surface abundances contain valuable information on what is happening in the unseen
interior of the star.
![]() The determination
of chemical abundances from astronomical observations requires atomic
data: the probabilities for absorption
of light by each species of atom at its set of characteristic wavelengths. These probabilities are usually given in a
form called, for historical reasons, the “oscillator strength.”
The determination
of chemical abundances from astronomical observations requires atomic
data: the probabilities for absorption
of light by each species of atom at its set of characteristic wavelengths. These probabilities are usually given in a
form called, for historical reasons, the “oscillator strength.”
![]() The Fast-Ion-Beam Laser Lab at Western:
The Fast-Ion-Beam Laser Lab at Western:
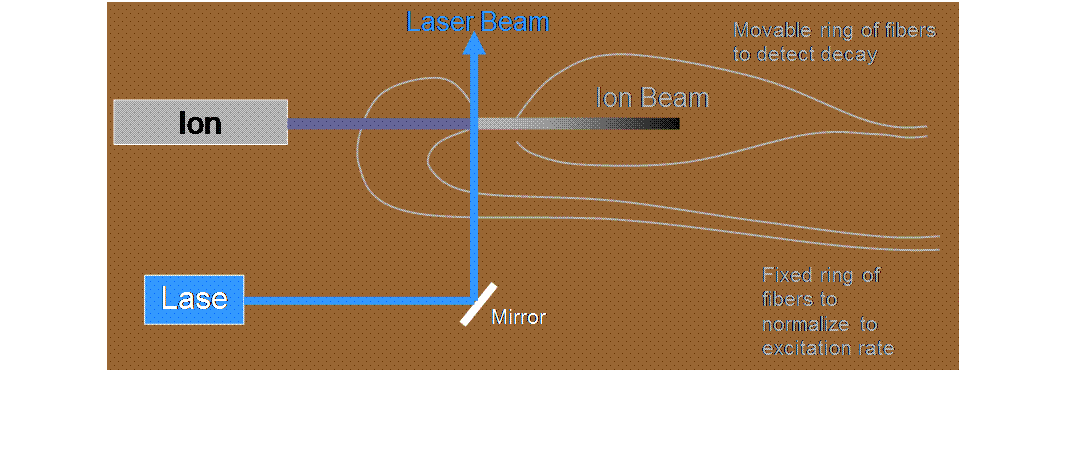
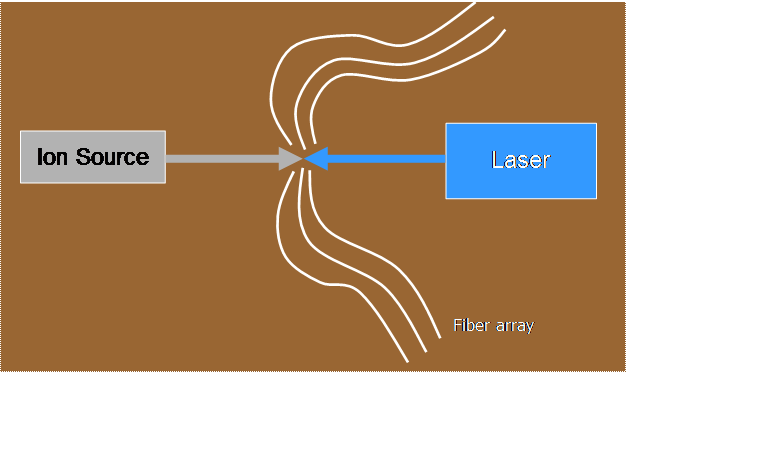
![]() We produce a 10-keV
beam of ions traveling in vacuum in our accelerator facility
We produce a 10-keV
beam of ions traveling in vacuum in our accelerator facility
![]() A continuous-wave
dye laser (pumped by an argon-ion laser) excites the ions to a particular
unstable state, from which they can decay to various possible states of lower
energy, emitting a photon while still traveling at high speed (1.5 x 105
m/s)
A continuous-wave
dye laser (pumped by an argon-ion laser) excites the ions to a particular
unstable state, from which they can decay to various possible states of lower
energy, emitting a photon while still traveling at high speed (1.5 x 105
m/s)
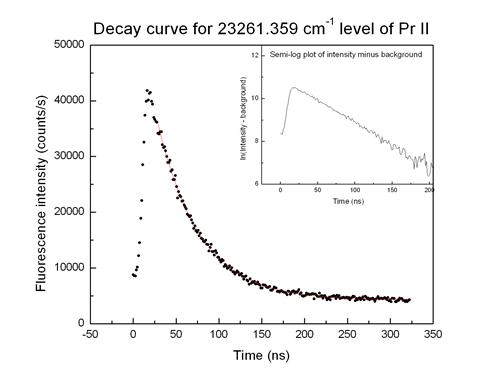
![]() From the curve of
light emitted as a function of distance “downstream” from the excitation region
(which corresponds to time since excitation) we determine the average lifetime
of the unstable state, typically tens to hundreds of nanoseconds
From the curve of
light emitted as a function of distance “downstream” from the excitation region
(which corresponds to time since excitation) we determine the average lifetime
of the unstable state, typically tens to hundreds of nanoseconds
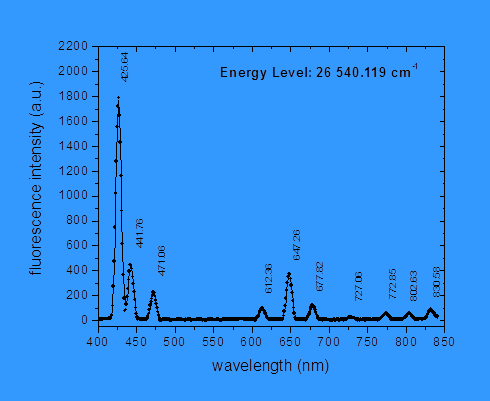
![]() From spectroscopic
measurements of the relative intensities of the light emitted in the various
possible transitions, together with the lifetime of the upper state, we then
determine the probability per second of emitting a photon in each of the
possible transitions. This quantity is
called the “Einstein A coefficient.”
From spectroscopic
measurements of the relative intensities of the light emitted in the various
possible transitions, together with the lifetime of the upper state, we then
determine the probability per second of emitting a photon in each of the
possible transitions. This quantity is
called the “Einstein A coefficient.”
|
|
|
![]() A simple
relationship between the probabilities for spontaneous emission and for
absorption, first derived by Einstein, allows us to calculate the quantities
needed by astrophysicists: the
oscillator strengths.
A simple
relationship between the probabilities for spontaneous emission and for
absorption, first derived by Einstein, allows us to calculate the quantities
needed by astrophysicists: the
oscillator strengths.