Research
- Research Overview
- Organo Metallic Chemical Vapor Deposition
- Optical Response of Gold Nanoparticle Clusters
- Evanescent Wave Analytical Tools
- Surface Functionalisation
- Detection of Optically Active Molecules
- Optical Tweezers in Evanescent Fields
- Evanescent Microscopy and Spectroscopy
- Research Opportunities
- Research Group
- Former Coworkers and Students
- Collaborators
- Publications
Contact Information
Prof. Silvia Mittler
Physics & Astronomy 240
(519) 661-2111 x.88592
smittler@uwo.ca
FAX: (519) 661-2033
Organo Metallic Chemical Vapor Disposition (OMCVD)
In this current research project both gold and palladium OMCVD precursors are synthesised and used to deposite ultra thin films of palladium/palladium ions or pancake-shaped gold nanoparticles. The main interest is to use these new metal structures for binding chemical and biological recognition sites via thiol (-SH) or sulfide bonds. On the other hand the nucleation and growth process of these new kind of nanoparticles is still not fully understood.
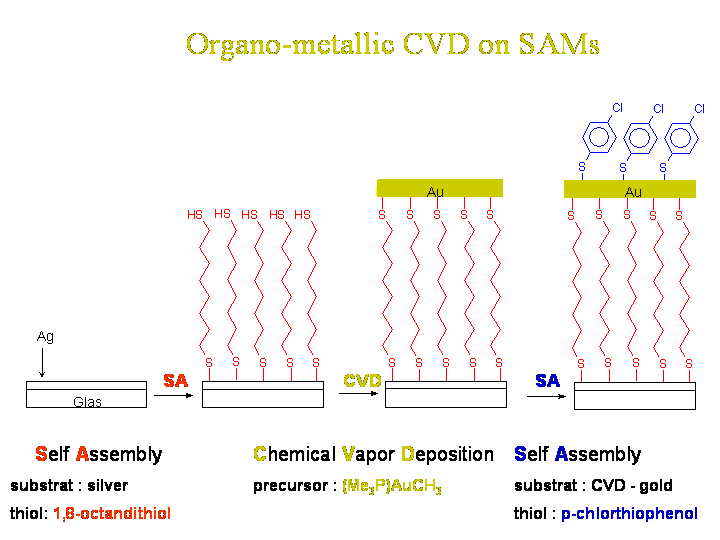
Templated deposition of metals - here an example with gold - via a precursor molecule in a chemical vapor deposition process onto self-assembled monolayers (SAMs) carrying a function - here -SH - being able to bind the metal to the surface or catalyse the deposition. A second SAM is self-assembled on top of the deposited metal layer in a further step.
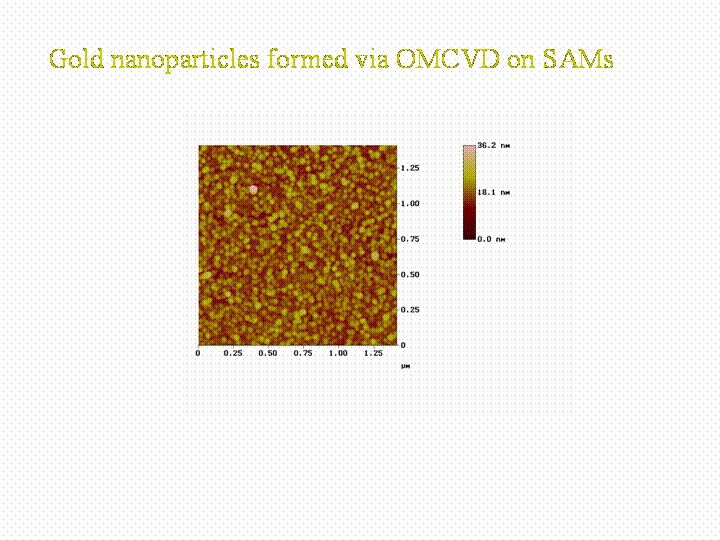
In the case of our gold precursor we form gold nanoparticles on the SAM surface. The form of the nanoparticles depend on the density of -SH groups in the surface. By having a 100% coverage with -SH groups very falt particles are formed, by decreasing the -SH density due to mixing -CH3 head groups in, the particles grow in a less flat manner.
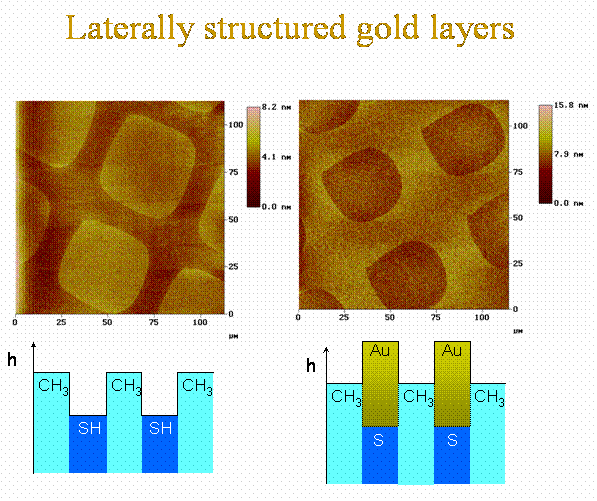
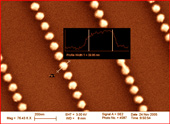
Contrast inversion images taken by an AFM due to selective growth of gold by organo-metallic chemical vapor deposition onto -SH rich surfaces of a chemical laterally structured self-assemled monolayer of organic molecules with different lengths.
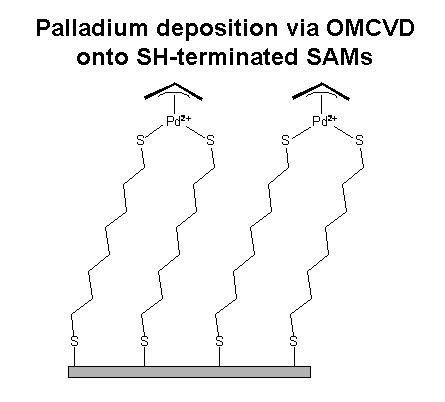
In the case of our palladium prercursor, we can bind Pd2+ ions to the SAM. This Pd2+ is able to bind a thiol with a function. This concept should enable us to develop a "metalic glue" for thiol molecules on glassy integrated optical devices without enhancing the optical losses.
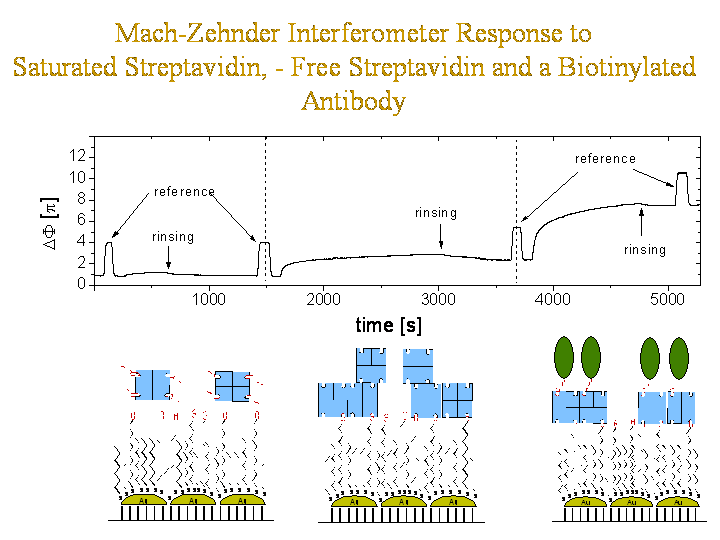
An optically integrated Mach-Zehnder interferometer was fabricated carrying gold nanoparticles on its active surfaces. These nanoparticles were functionalized with a biotinilated SAM in order to bind the protein streptavidin. First one interferometerarm was rinsed with streptavidin previously saturated with biotin to test for unspecific binding. In a second experiment free straptavidin was used to bind to the exposed biotin sites. In a third experiment biotinylated antibodies were rinsed along one interferometer arm. Here the biotin bilds into the free and exposed binding pockets of the immobilised sytreptavidin.